There are various calculation and formulas required for the Calculation of MACO (Maximum allowable Carry Over), PDE (Permitted Daily Exposure) and NOEL (No Observed Effect Level) in Cleaning Validation.
All these formulas are helpful in implementing the correct cleaning validation matrix and plan at facility. Herewith are detailed full proof formulas which will build error free design of Cleaning Validation Matrix and Calculation of MACO/PDE
Permitted Daily Exposure (PDE):
PDE is the amount of a specific substance for which the occurrence of an adverse effect is unlikely in an individual.
Formula for Calculation PDE:
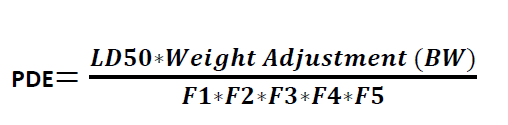
Where
LD50 = the 50 % of lethal dose of the target residue in an animal, typically in mg/kg of body weight (by appropriate route of administration)
BW = body weight of the patient taking next product
F1 = A factor (values between 2 and 12) to account for extrapolation between species
F2 = A factor of 10 to account for variability between individuals
F3 = A factor 10 to account for repeat-dose toxicity studies of short duration, i.e., less than 4-weeks.
F4 = A factor (1-10) that may be applied in cases of severe toxicity, e.g. non-genotoxic carcinogenicity, neurotoxicity or teratogenicity
F5 = A variable factor that may be applied if the no-effect level was not established. When only an LOEL is available, a factor of up to 10 could be used depending on the severity of the toxicity.
Formula for Calculation MACO from PDE:
MACO
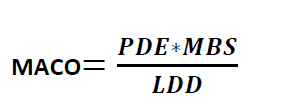
MACO = Maximum Allowable Carry Over
MBS = Minimum Batch Size of the next Product
LDD = Largest Daily Dose of the Next Product to be Manufactured in the same equipment
NOEL (No-Observed-Effect Level)
No-Observed-Effect Level: The highest dose of substance at which there are no biologically significant increases in frequency or severity of any effects in the exposed humans or animals.
Formula for Calculation NOEL:
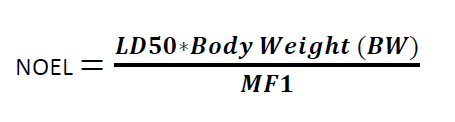
Where
NOEL is the No Observed Effect Level
LD50 = the 50 % of lethal dose of the target residue in an animal, typically in mg/kg of body weight (by appropriate route of administration)
BW = body weight of the patient taking next product
MF1 = modifying factor or factors selected by the toxicologist (cumulative modifying factors selected are generally not more than 1000)
[Reference PDA Technical Report No. 29 (revised)-Points to Consider for Cleaning Validation, Point 5.3.2.2-Toxicity Calculations Based on LD50 Data]
Formula for Calculation MACO from NOEL:
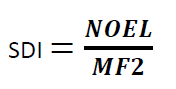
Where
SDI = Safe Daily Intake of the residue
NOEL = No Observed Effect Level
MF2 = modifying factor or factors selected by the toxicologist (cumulative modifying factors selected are generally not more than 1000)
[Reference PDA Technical Report No. 29 (revised)-Points to Consider for Cleaning Validation, Point 5.3.2.2-Toxicity Calculations Based on LD50 Data]
MACO (Maximum Allowable Carry Over)
Formula for Calculation of MACO by Using SDI:
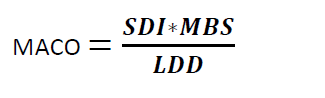
Where
MACO = Maximum Allowable Carry Over
SDI = Safe Daily Intake of the residue
MBS = Minimum Batch Size of the next Product
LDD = Largest Daily Dose of the Next Product to be Manufactured in the same equipment
[Reference PDA Technical Report No. 29
(revised)-Points to Consider for Cleaning Validation, Point 5.3.2.2-Toxicity
Calculations Based on LD50 Data]
Dose Criteria
MACO (Maximum Allowable Carry Over)
Formula for Calculation of MACO by Using Dose Criteria:
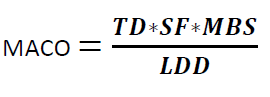
Where
TD is the Minimum Therapeutic Dose of Product A
MACO = Maximum Allowable Carry Over
MBS = Minimum Batch Size of the next Product B
LDD = Largest Daily Dose of the Next Product to be Manufactured in the same equipment
10 PPM Criteria
Formula for Calculation of MACO by Using 10 PPM Criteria:
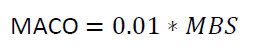
Where
0.01 is equivalent to 10 PPM
MACO = Maximum Allowable Carry Over
MBS
= Minimum Batch Size of the next Product B in ml
Calculation for Concentration Limits:
Calculation for Swab Limits:
Swab Limits (µg/Swab) or PPM
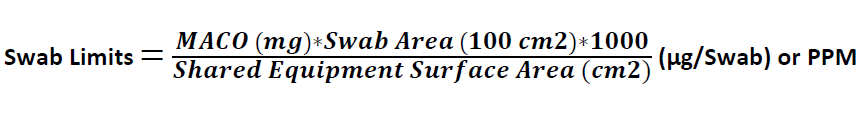
Where
MACO is derived from the above equations
100 cm2 = Swab Area is Standard as 100 cm2
Shared equipment surface area is the shared area of Product B
1000 is the conversion factor from mg to µg / PPM
Calculation for Rinse Limits:
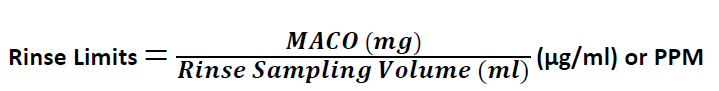
[Reference PDA Technical Report No. 29 (revised)-Points to Consider for Cleaning Validation, Point 5.6 –Limits in Protocol Samples]
Conclusion
Cleaning Validation in Pharmaceutical Industry should be designed very carefully and the calculation for designing matrix plays a very important role. A small calculation error can leads to the big gap in the system. Therefore, it should be performed very carefully. As on date there are ready to install software available for executing cleaning validation calculation, however, it has to be evolved a lot more.

Raman is a versatile experienced Bio-pharmaceutical professional with more than 12 year of experience in Sterile and Non-Sterile Formulations. Raman is working in different aspects of Sterile Validations and designing Pharmaceuitcals Quality Systems for the next century. He is a versatile and tech savvy professional who believe the Quality is the foundations of Growing Organizations








