Trends of the Pharmaceutical Industry is changing with huge pace and there is strong need of creating the Quality Assurance who has acquired skills in all dimensions so as to combat and sustain in this highly volatile environment with high level of competition. In this article we will discuss ten such skills which are the need of time.
1. Process Understanding
Understanding about the process under manufacturing has been an important factor which helps is the robust investigation, identification of Root causes and designing of the corrective actions.
Poor understanding of the process will leads to the underlining factors which will remain unexplored during such scenarios.
This can be achieved by the continuous learning and knowledge sharing with the team. Protocol of the Resource Management should be there which will ensure that the personnel are getting acquainted with the Process continuously and also updated as and when there is any update.
2. Operating Principle
Pharmaceutical Industry is equipped with multiple critical equipment such as Manufacturing equipment, support utilities and testing systems.
All these equipment are working with a scientific principle which has to be taught to the team while allowing them to operate through. There are two main reasons for this:
Critical equipment with high asset value and highly sensitive has to be handled with care
Knowing the scientific understanding behind the equipment before working upon it will help the operator and other user to have ease of process operation and troubleshooting as and when required.
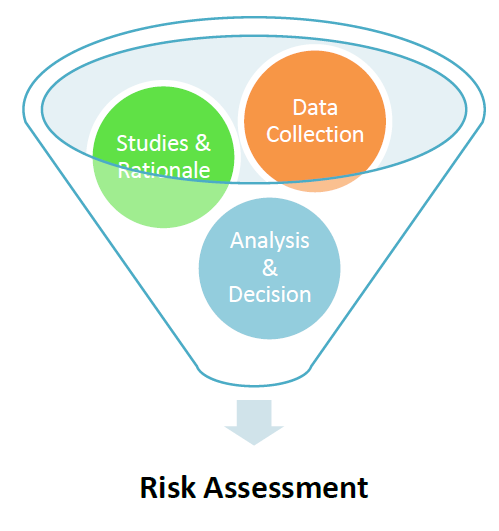
3. Risk Assessment
ICH Q9 titled Risk Assessment, has changed the scenario of the Pharmaceuticals Industry and has given a great tool to the Industry to prevent any unwanted failures.
Risk Assessment has the following two advantages:
– Helps to identify proactively any risk associated with process, equipment or system
– It helps to rationalize any statement or decision which has been taken based upon the scientific understanding and data analytics
Above two factors are quite very much related to each pharmaceutical professional working in Quality Unit. Therefore its understanding will enable them to handle the changes, processes and system and helps in taking the adequate decisions, mitigations and deriving the robust action plans for tackling the change, process or system.
4. Investigative Skills
Look at some popular keywords – Poor Investigation, Root cause not identified, repeated deviation, repeated failures, inadequate rationalization, etc.
These keywords have become important words of the FDA warning letters nowadays.
Going into reality, they are very much true also, in the competitive business scenario there is very less importance given to such issues. Failures are not considered as an opportunity for learning and reasons behind their existence remains unexplored and hidden
Consequently, these are propagated in the Investigators findings and audit reports.
So the importance of investigative skills and writing scientific investigation which prevent repeated failures shows it utmost increasing importance.
5. Guidance Understanding
Yes, pharmaceuticals Industry is driven by Drugs and Cosmetic Act and Regulatory Guidelines and updates.
Multiple regulatory bodies such as USFDA, WHO, PDA, PIC/S, EMEA, ISO, ISPE, ICH, MHRA etc. are publishing multiple guidelines in almost every segment of pharmaceutical industry which helps professional to upgrade the standards of the industry to generate the drugs of high Quality.
These guidelines are the tools for the proactive improvements of the system and complying to the increasing regulatory expectations.
Having the understanding of these guidelines enable and prepares the Industry to future challenges. Therefore shows its importance.
6. CAPA Designing
Organizational approach to the problems is observed by the regulators through the CAPA designing procedures & the extent of the actions taken to eliminate the problem completely. It’s like “Hitting the Buzzer” and responding first.
In case regulators observe that the Organizations are not responding adequately and on time, it is considered as an inadequate approach and seriousness towards the issues.
CAPA basically comprises of following three steps:
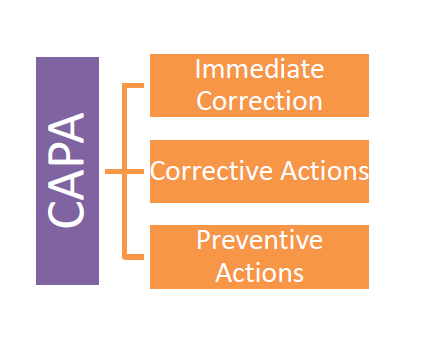
Understanding each step and its significance is very much important and Organizations QA Team must understand them carefully and design the CAPA to each failure with properly so that it clearly reflects organizational approach and seriousness.
7. Communication & Analytical Skills
Good Communications skills are very much important. This is required for the team development and trainings. Proper Analysis of Issues and communication of QA decisions plays a major role in QA functions.
Also these skills are the key factor while addressing the concerns of the regulators during the Inspections. Any communication which does not helps the regulators/auditors to understand may leads to gaps or deficiency.
8. Data Analytics:
Data is the currency of 21st Century. One who understands the data and makes its correct interpretation has lots of importance in the upcoming decades.
Data enables in decision making and future forecast. Data is considered as one major area in pharmaceutical sector and most of the elements are data based such as Process Validation, APQR, Trend, Control Limits, AMV, Quality Matrix etc.
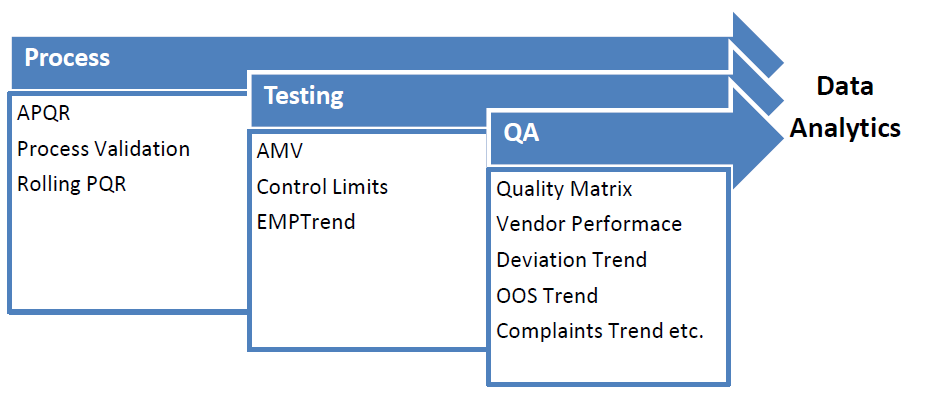
All comprises of data science. Having understanding of this data science is the logics behind all these tools adopted by QA to understand the process and its control.
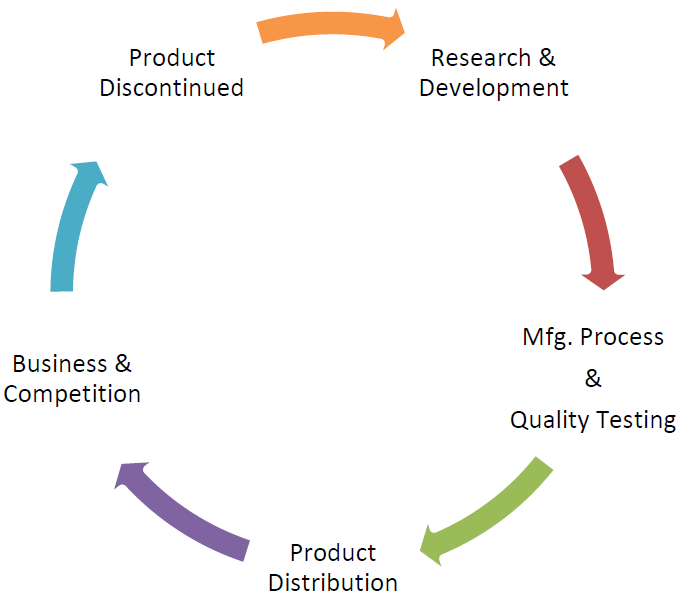
9. Business Understanding
Business is a process which has its own cycle and time phrase. Product Lifecycle approach is the expectation of regulators nowadays. Product development to Product discontinuation all stages has relevant importance and Quality by Design has to be integrated at all points of cycle. Quality alone cannot be integrated or built at manufacturing phase; however it is the combines’ impact of all functions.
Product robustness shall be established during the product development phase which forms a major part of Quality Assurance; however the product robustness is given low preference in terms of building robust process which has broad design space and product quality attributes does not alter with minor processes changes.
Therefore, it is very important to design Business Goal strategies very carefully and energy with sufficient amount should be consumed to get these goals achieved.
“PQS should act as an enabler”
Below statement from ICH Q10 titled “Pharmaceutical Quality System” itself has lots of meaning which support the above statement
Section 1.6 of ICH Q10 describes about Enablers
Use of knowledge management and quality risk management will enable a company to implement ICH Q10 effectively and successfully. These enablers will facilitate achievement of the objectives Operation Management & Enabling Operations which includes:
- Product Realization
- State of Control
- Continual Improvement
10. Employee Engagement
“Employee engagement is Employee Performance”.
Productivity depends upon the engagement of the Employee with the Organization. Research proved that engaged employees are more productive.
Refer the article “7 Strategies for Employee Engagement” for more information how to engage the employees.
Employee Engagement can be brought with adequately handling the team and developing them continuously so that they can chase their personnel goals. Organization should ensure that the team members are being prepared for the future challenges. A Manager or a Team Leader should ensure that the team members of department and cross-functional team are not encountered with Conflicts and polarization behaviors as they can lead to the drifts and hamper the employee engagement and productivity.

Raman is a versatile experienced Bio-pharmaceutical professional with more than 17 year of experience in Sterile and Non-Sterile Formulations. Raman is working in different aspects of Sterile Validations and designing Pharmaceuitcals Quality Systems for the next century. He is a versatile and tech savvy professional who believe the Quality is the foundations of Growing Organizations









